06 April, 2023
News
Crescom’s MediAI-BA, a company specializing in artificial intelligence medical image analysis solutions, has obtained medical device approval from Malaysia’s MDA.
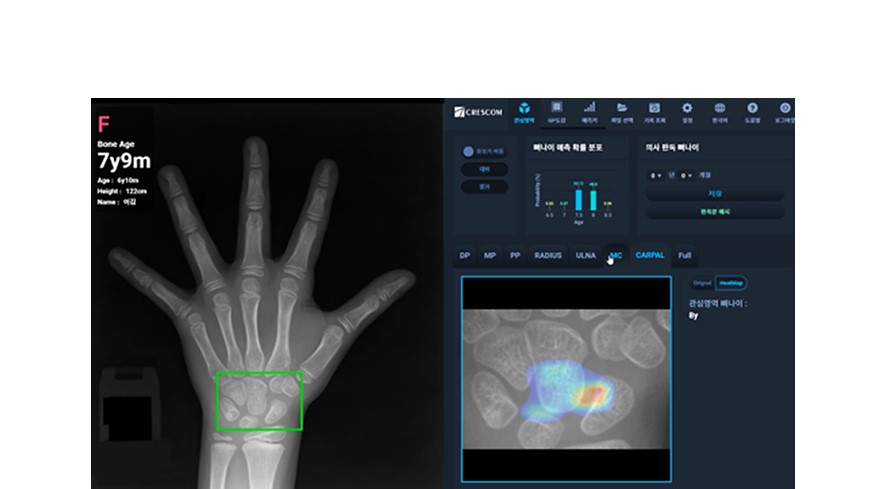
(Photo: artificial intelligence bone
age analysis software, MediAI-BA _ ⓒ Crescom Co., Ltd.)
(Date: April 6, 2023)
Crescom
(CEO Jae Joon Lee), a company specializing in the automatic analysis of
artificial intelligence medical images, announced that it had obtained medical
device registration for MediAI-BA from Malaysia MDA (Medical Device Authority,
Ministry of Health Malaysia). The acquisition of the Malaysian certificate is
significant as it provides an opportunity to enter the Malaysian market, which
is continuously growing with a significantly high GDP growth rate among ASEAN
countries.
MediAI-BA
is a hybrid artificial intelligence growth plate analysis solution that
combines the strengths of existing bone age reading techniques (GP and TW3)
while addressing their weaknesses. This solution assists medical staff in
accurate and quick diagnosis by increasing the efficiency and accuracy of growth
plate bone age analysis in children and adolescents. Currently, about 250
medical institutions actively use MediAI-BA with very high satisfaction, and
the number of institutions using it is rapidly increasing.
In
addition to MediAI-BA, Crescom offers other artificial intelligence medical
solutions specialized in musculoskeletal image analysis: MediAI-FX (automatic
detection of fracture regions and analysis of fracture probability, including
difficult-to-read scaphoid fractures), MediAI-OA (knee arthritis severity
automatic analysis solution), and MediAI-AS (ankylosing spondylitis automatic
evaluation solution). Crescom's technology has confirmed the excellent
performance of these solutions.
Meanwhile,
Crescom's MediAI-BA obtained certification from the Ministry of Food and Drug
Safety(MFDS) and ISO13485:2016 in 2020 prior to obtaining medical device
registration in Malaysia. CEO Jae Joon Lee said, “Following this Malaysian
authorization, we intend to contribute to expanding access to innovative high-quality
medical services in Southeast Asia, including Singapore, where the
authorization process is currently underway. Furthermore, we are expanding our
export products, including knee arthritis severity automatic analysis
artificial intelligence solution, and pushing for entry into the American
market as well as Asia."
-
 Crescom’s wrist fracture detection reading artificial intelligence solution, MediAI-FX, was published in an international journal.22 May, 2023
Crescom’s wrist fracture detection reading artificial intelligence solution, MediAI-FX, was published in an international journal.22 May, 2023 -
 Crescom’s MediAI-BA, a company specializing in artificial intelligence medical image analysis solutions, has obtained medical device approval from Malaysia’s MDA.06 April, 2023
Crescom’s MediAI-BA, a company specializing in artificial intelligence medical image analysis solutions, has obtained medical device approval from Malaysia’s MDA.06 April, 2023 -
 Signed a joint sales contract with Ahngook Pharm. For Crescom’s artificial intelligence bone age analysis solution08 March, 2023
Signed a joint sales contract with Ahngook Pharm. For Crescom’s artificial intelligence bone age analysis solution08 March, 2023